Abstract
Objective: Fanconi anemia (FA) is the most common inherited bone marrow failure syndrome associated with multiple congenital abnormalities and predisposition to malignancies, resulting from mutations in one of the 22 known FA genes (FANCA to W). The proteins encoded by these genes participate in DNA repair pathway (the FA pathway) for endogenous aldehyde damage. Compared to the situation in the US or Europe, the number of Japanese FA patients with genetic diagnosis was relatively limited. In this study, we reveal the genetic subtyping and the characteristics of mutated FA genes in Japanese population and clarify the genotype-phenotype correlations.
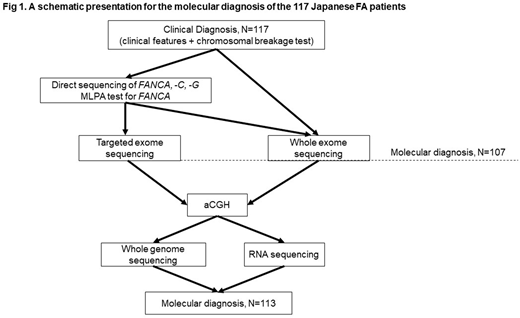
Results: We studied 117 Japanese FA patients from 103 families (1996 to 2018). The diagnosis of FA was confirmed on the basis of chromosomal breakage tests and clinical features. Molecular diagnosis was obtained in 107 (91.5%) of the 117 patients through direct sequencing of FANCA and FANCG, MLPA analysis for FANCA, targeted exome sequencing (targeted-seq), and whole exome sequencing (WES) analysis (Figure 1). To provide genetic subtyping for the 10 unclassified cases, we tried to apply various technologies. Array CGH revealed large deletions in two FA-B and one FA-T cases. Whole genome sequencing and RNA-sequencing analysis identified splicing site or aberrant splicing mutations among three cases (one FA-B, one FA-C, and one FA-N). Collectively, 113 (97%) of Japanese 117 FA patients were successfully subtyped and a total of 219 mutated alleles were identified. FA-A and FA-G accounted for the disease in 58% and 25% of FA patients, respectively, whereas each of the other complementation groups accounted for less than 5% of FA cases. FANCB was the third most common complementation group (n=4) and only one FA-C case was identified in Japanese FA patients.
In the 68 FA-A patients, we identified 130 mutant alleles that included 55 different FANCA variants (17 nucleotide substitutions, 16 small deletions/insertions, 12 large deletions, 1 large duplication and 9 splice site mutation). FANCA c.2546delC was the most prevalent (41/130 alleles; 32%). In the 29 FA-G patients, 57 mutant alleles were identified and seven different FANCG variants were detected. FANCG c.307+1G>C and 1066C>T accounted for most of FANCG mutant alleles (49/57; 88%) in the Japanese FA-G patients. The three hotspot mutations (FANCA c.2546delC, FANCG c.307+1G>C and c.1066C>T) existed at low prevalence (0.04-0.1%) in the whole-genome reference panel of 3554 Japanese individuals (3.5KJPN, Tohoku Megabank). Consistent with the paucity of the FA-C patients as opposed to the previous report (Blood 2000), the FANCC IVS4+4A mutation was absent in the 3.5KJPN database.
We were able to examine the hematological outcomes in a subset of our cases (52 FA-A and 23 FA-G). Interestingly, the FA-G patients developed bone marrow failure (BMF) at a significantly younger age than FA-A patients (median age at onset of BMF: 3.1 years vs 5 years). Furthermore, the patients with the FANCA c.2546delC mutation had an increased risk of developing myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML), compared to FA-A patients without the mutation.
In the rare complementation groups of FA, two FA-B cases with complete loss of FANCB gene and one FA-I patient with N-terminal premature termination codons revealed severe somatic abnormalities, consistent with VACTERL-H association. Two FANCD1 (BRCA2) patients and one FANCN (PALB2) patients did not experience bone marrow failure but developed early-onset malignancies (immature teratoma, T-lymphoblastic lymphoma, adenosquamous lung carcinoma, Wilms tumor).
Conclusion: This is the largest series of subtyped Japanese FA patients to date and the results would be useful for future clinical management. To provide molecular diagnosis for FA in Japan, we suggest to start with PCR-direct sequencing of the three common mutations (FANCA c.2546delC, FANCG c.307+1G>C and FANCG c.1066C>T) along with MLPA assay for FANCA. These analyses would enable the identification of about 50% of the mutant alleles. For the rest of the cases, WES or targeted-seq analysis should be useful, however, large deletions and aberrant splicing need to be kept in mind.
Takaori-Kondo:Pfizer: Honoraria; Novartis: Honoraria; Celgene: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria; Janssen Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.